Ultrafast fabrication of nickel sulfide film on Ni foam for efficient overall water splitting†
Abstract
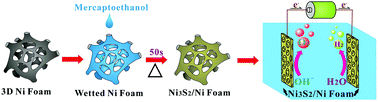
Development of low-cost, high performance and stable non-noble electrocatalysts with both hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) activities for overall water splitting is essential for future energy supply. Herein, for the first time, a facile and ultrafast synthetic method has been reported to fabricate nickel sulfide (Ni3S2) films on Ni foam (Ni3S2/NF) as efficient bifunctional electrodes for overall water splitting through direct dropping of mercaptoethanol solution followed by annealing at 300 °C for only 50 s. Thanks to the integrated three-dimensional (3D) configuration, the obtained Ni3S2/Ni foam exhibits excellent activity and stability for HER and OER with low overpotentials of 131 and 312 mV, respectively, to attain a current density of 10 mA cm−2 in alkaline media. Ni(OH)x species formed on the Ni3S2 surface serves as the actual catalytic site during OER reaction. Given the well-defined bifunctionality, an overall water-splitting device using two identical Ni3S2/NF electrodes delivers a current density of 10 mA cm−2 at a low cell voltage of 1.68 V in an alkaline water electrolyzer. This approach is promising as a simple method for depositing a wide range of useful transition metal sulfide electrocatalysts on corresponding metal substrate bifunctional electrodes for overall water splitting, shedding some light on the development of functional materials in energy chemistry.


 Please wait while we load your content...
Please wait while we load your content...