Phase control in the colloidal synthesis of well-defined nickel sulfide nanocrystals†
Abstract
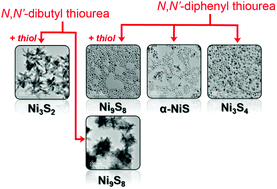
Morphologically well-defined colloidal nanocrystals of Ni3S4, NiS, Ni9S8, and Ni3S2 were independently prepared through a solution-phase synthesis using N,N′-disubstituted thioureas as the sulfur precursor. Synthetic control over phase and composition of the resulting colloidal nickel sulfide nanocrystals was achieved by primarily adjusting the reactivity of substituted thioureas as well as tuning the key reaction parameters of temperature and precursor ratio. In general, the more reactive N,N′-diphenyl thiourea yields more sulfur-rich phases (Ni3S4 and NiS) while less reactive N,N′-dibutyl thiourea yields sulfur-poor phases (Ni9S8 and Ni3S2). This phase control can be further tuned through the use of 1-dodecanethiol as an important secondary reactivity-directing agent. In the presence of 1-dodecanethiol, nanocrystals of more sulfur-deficient phases are prepared, while in the absence of 1-dodecanethiol, more sulfur-rich phases are prepared. Under the most sulfur-rich synthetic conditions (i.e., with N,N′-diphenyl thiourea and no thiol) a phase progression from Ni3S4 to the α-NiS and β-NiS phases was observed upon an increase in reaction temperature and sulfur-to-nickel precursor ratio. This study establishes, for the first time, a systematic evaluation of factors that simultaneously control the phase and yield well-defined nickel sulfide nanocrystals.


 Please wait while we load your content...
Please wait while we load your content...
