Multiscale porous molybdenum phosphide of honeycomb structure for highly efficient hydrogen evolution†
Abstract
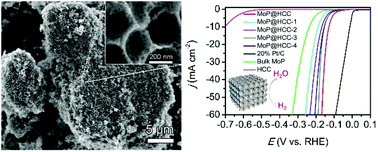
The hydrogen evolution reaction (HER) based on electrochemical water splitting is considered a promising strategy to produce clean and sustainable hydrogen energy. Searching for non-noble metal based electrocatalysts with high efficiency and durability toward the HER is vitally necessary. In this work, we report a novel method for synthesizing molybdenum phosphide (MoP) supported on multiscale porous honeycomb carbon (MoP@HCC) and the application of this catalyst material in acidic media for water electrolysis. Due to the unique structure of the catalyst material, the as-prepared MoP@HCC shows remarkable electrocatalytic activity and stability in 0.5 M H2SO4 aqueous solution. The hybrid catalyst could deliver a current density of 10 mA cm−2 at a low overpotential of 129 mV, with an onset overpotential of 69 mV and a Tafel slope of 48 mV dec−1, outperforming most of the current noble-metal-free electrocatalysts. This study demonstrates an effective way for multiscale control of the MoP structure via overall consideration of the mass transport, and the accessibility, quantity and capability of active sites toward the HER.


 Please wait while we load your content...
Please wait while we load your content...