High electrocatalytic performance inspired by crystalline/amorphous interface in PtPb nanoplate†
Abstract
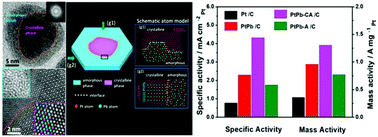
Nanoscale PtPb catalysts with core–shell structure have been actively explored in recent years owing to their outstanding catalytic activity. We report on a new class of PtPb nanoplate (NP) catalyst with a novel structure realized by ion irradiation modification, which contains an interface formed by a crystalline phase and an amorphous phase simultaneously in an annular state. Significantly, the PtPb NP with the new structure shows superior catalytic activity towards the methanol oxidation reaction (MOR). The specific activity of PtPb NPs with the new structure reaches 4.32 mA cm−2 towards the MOR and the mass activity reaches 1.31 A mg−1, which is 1.9-fold and 1.4-fold greater than those for the original crystalline PtPb NPs, respectively. The outstanding catalytic activity could be attributed to the presence of the interface between a crystalline phase and an amorphous phase with a special electronic structure created by ion irradiation. Density functional theory calculations reveal that the novel interface activates the C–H and O–H bonds, leading to high electrocatalytic activity, and optimizes the adsorption of hydroxyl and intermediates on the surface to facilitate the oxidation reaction. The novel structure with an interface formed by a crystalline phase and an amorphous phase opens up a new approach to improve electrocatalytic activity.


 Please wait while we load your content...
Please wait while we load your content...