A porous nickel cyclotetraphosphate nanosheet as a new acid-stable electrocatalyst for efficient hydrogen evolution†
Abstract
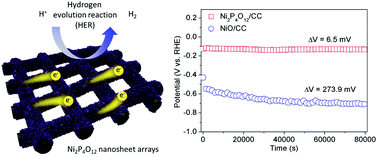
The stability of non-precious metal-based electrocatalysts for the acidic hydrogen evolution reaction (HER) is of great importance. Here, we have used nickel cyclotetraphosphate (Ni2P4O12) nanosheet arrays as a HER electrocatalyst for the first time. The Ni2P4O12 arrays were obtained through a facile low-temperature phosphorylation process and possess superior HER catalytic activities and stability in acid. The Ni2P4O12 delivers a small overpotential of 131.8 mV at −10 mA cm−2 and a low Tafel slope of 47.8 mV dec−1 in 0.5 M H2SO4, comparable to most of the non-precious metal-based catalysts. Importantly, the Ni2P4O12 shows a negligible potential change (6.5 mV) over 80 000 s continuous testing in acid. The remarkable catalytic performances of Ni2P4O12 are mainly attributed to the inductive effect of P4O124− and its polymer-like structure, promoting it as a potential acid-stable HER electrocatalyst.


 Please wait while we load your content...
Please wait while we load your content...