Bulk-terminated or reconstructed Fe3O4(001) surface: water makes a difference†
Abstract
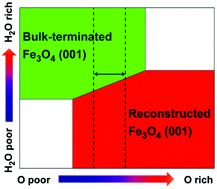
Surfaces and their interaction with water play an important role in most of materials’ applications. Magnetite has attracted continued interest in the fields of catalysis, spintronic devices, magnetic resonance imaging (MRI) and drug delivery. In this work, water adsorption and its effect on the stability diagram and on the electronic structure of the Fe3O4(001) surface are investigated by hybrid density functional theory calculations combined with an ab initio atomistic thermodynamic approach. We span a wide range of gaseous O2 and vapor H2O partial pressures. At low water pressure, a reconstructed SCV surface model is confirmed to be the most stable model at common working O2 partial pressures. However, at high water coverage, an unexpected stability inversion is observed that makes the hydrated bulk-terminated DBT surface the most favored. These results open up new horizons in Fe3O4 surface chemistry when working in an aqueous environment and are of key importance to develop rational strategies to surface engineering for high performance Fe3O4 nanomaterials.


 Please wait while we load your content...
Please wait while we load your content...