Re-evaluating how charge transfer modifies the conformation of adsorbed molecules†
Abstract
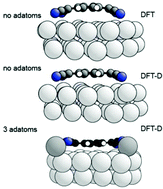
The archetypal electron acceptor molecule, TCNQ, is generally believed to become bent into an inverted bowl shape upon adsorption on the coinage metal surfaces on which it becomes negatively charged. New quantitative experimental structural measurements show that this is not the case for TCNQ on Ag(111). DFT calculations show that the inclusion of dispersion force corrections reduces not only the molecule-substrate layer spacing but also the degree of predicted molecular bonding. However, complete agreement between experimentally-determined and theoretically-predicted structural parameters is only achieved with the inclusion of Ag adatoms into the molecular layer, which is also the energetically favoured configuration. The results highlight the need for both experimental and theoretical quantitative structural methods to reliably understand similar metal–organic interfaces and highlight the need to re-evaluate some previously-investigated systems.


 Please wait while we load your content...
Please wait while we load your content...