Boosting photocatalytic overall water splitting by Co doping into Mn3O4 nanoparticles as oxygen evolution cocatalysts†
Abstract
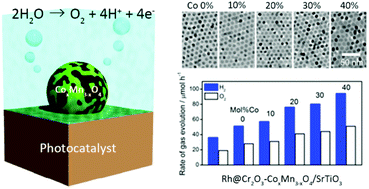
The effect of cobalt doping into a manganese oxide (tetragonal spinel Mn3O4) nanoparticle cocatalyst up to Co/(Co + Mn) = 0.4 (mol/mol) on the activity of photocatalytic water oxidation was studied. Monodisperse ∼10 nm CoyMn1−yO (0 ≤ y ≤ 0.4) nanoparticles were uniformly loaded onto photocatalysts and converted to CoxMn3−xO4 nanoparticles through calcination. 40 mol% cobalt-doped Mn3O4 nanoparticle-loaded Rh@Cr2O3/SrTiO3 photocatalyst exhibited 1.8 times-higher overall water splitting activity than that with pure Mn3O4 nanoparticles. Investigation on the band structure and electrocatalytic water oxidation activity of CoxMn3−xO4 nanoparticles revealed that the Co doping mainly contributes to the improvement of water oxidation kinetics on the surface of the cocatalyst nanoparticles.


 Please wait while we load your content...
Please wait while we load your content...