Hydrogen evolution reactions boosted by bridge bonds between electrocatalysts and electrodes†
Abstract
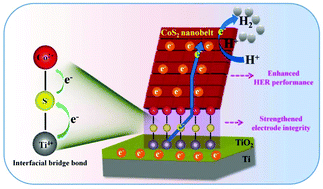
The interfacial interactions between nanostructured electrode materials and electrodes play an important part in the performance enhancement of electrochemical energy devices. However, the mechanism of interfacial interactions, as well as its influence on device performance, still remains unclear and is rarely studied. In this work, a CoS2 nanobelt catalyst assembled on Ti foil (CoS2 nanobelts/Ti) is prepared through in situ chemical conversions and chosen as an example to probe the interfacial interactions between the CoS2 catalyst and the Ti electrode, and the correlation between the interfacial interaction and the hydrogen evolution reaction (HER) performance. By a series of characterization studies and analyses, we propose that interfacial bridge bonds (Ti–S–Co and Ti–O–Co) in a covalent form may exist in the CoS2 nanobelts/Ti as well as its precursor Co(OH)3 nanobelts growing on Ti foil, which is further supported by density functional theory (DFT) calculations. Moreover, as a binder-free electrocatalytic electrode, the CoS2 nanobelts/Ti shows boosted HER performance, including higher catalytic activity, and lower overpotential and Tafel slope, compared to its counterpart transformed from a solution-produced precursor. The HER performance enhancement is ascribed to the existence of interfacial bridge bonds that not only strengthen the electrode–catalyst mechanical integrity, but also serve as efficient charge transfer channels between the electrode and the catalyst, thus ensuring a stable and fluent electron transfer for the HER. Furthermore, the DFT calculations reveal that the CoS2 nanobelts/Ti catalyst with interfacial covalent interactions can facilitate the adsorption of H+ ions/H2 molecules and the desorption of H2 molecules for an accelerated HER. This work provides a new insight into the interfacial interactions between electrodes and electrode materials in electrochemical devices, and paves the way for the rational design and construction of high-performance electrochemical devices for practical energy applications.


 Please wait while we load your content...
Please wait while we load your content...