Interface engineering of a CeO2–Cu3P nanoarray for efficient alkaline hydrogen evolution†
Abstract
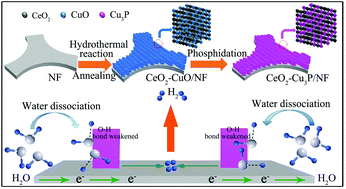
It is of great importance to design and develop highly active electrocatalysts for the hydrogen evolution reaction (HER) under alkaline conditions. In this work, we report the development of a CeO2–Cu3P nanoarray supported on nickel foam (CeO2–Cu3P/NF) as an excellent HER catalyst with the demand of an overpotential of only 148 mV to deliver a geometrical catalytic current density of 20 mA cm−2 in 1.0 M KOH. Remarkably, this catalyst also shows strong long-term electrochemical durability for at least 100 h with nearly 100% Faradaic efficiency. Density functional theory calculations reveal that the CeO2–Cu3P/NF hybrid has a lower water dissociation energy and a more thermo-neutral hydrogen adsorption free energy.


 Please wait while we load your content...
Please wait while we load your content...