Electro-oxidation of a cobalt based steel in LiOH: a non-noble metal based electro-catalyst suitable for durable water-splitting in an acidic milieu†
Abstract
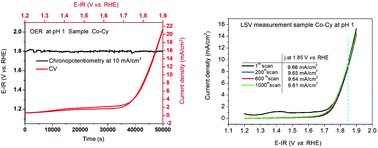
The use of proton exchange membrane (PEM) electrolyzers is the method of choice for the conversion of solar energy when frequently occurring changes of the current load are an issue. However, this technique requires electrolytes with low pH. All oxygen evolving electrodes working durably and actively in acids contain IrOx. Due to their scarcity and high acquisition costs, noble elements like Pt, Ru and Ir need to be replaced by earth abundant elements. We have evaluated a cobalt containing steel for use as an oxygen-forming electrode in H2SO4. We found that the dissolving of ingredients out of the steel electrode at oxidative potential in sulfuric acid, which is a well-known, serious issue, can be substantially reduced when the steel is electro-oxidized in LiOH prior to electrocatalysis. Under optimized synthesis conditions a cobalt-containing tool steel was rendered into a durable oxygen evolution reaction (OER) electrocatalyst (weight loss: 39 μg mm−2 after 50 000 s of chronopotentiometry at pH 1) that exhibits overpotentials down to 574 mV at 10 mA cm−2 current density at pH 1. Focused ion beam SEM (FIB-SEM) was successfully used to create a structure–stability relationship.


 Please wait while we load your content...
Please wait while we load your content...