A general strategy for synthesizing high-coercivity L10-FePt nanoparticles†
Abstract
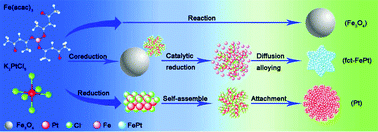
It is extremely desirable but challenging to develop a facile solution phase synthesis to directly prepare well-dispersed L10-FePt nanoparticles (NPs) to meet the requirements of advanced magnets in modern industry and information technology. Here, we report a novel strategy to synthesize hard magnetic L10-FePt NPs via controlled co-reduction of Fe(acac)3 and K2PtCl6 in the presence of oleylamine, in which effective control of the magnetic properties and chemical ordering of L10-FePt NPs was achieved by tuning the mole ratio of the precursors, reaction time and temperature. The highest coercivity of 10.5 kOe can be obtained for the NPs synthesized at 350 °C for 8 h, which is much higher than the coercivities reported by the previous studies on solution-synthesized FePt NPs without annealing or the third elemental additive. The reported one-pot synthesis of L10-FePt NPs may provide an ideal class of building blocks for magnetic energy applications.


 Please wait while we load your content...
Please wait while we load your content...