A novel mechanism for red emission carbon dots: hydrogen bond dominated molecular states emission†
Abstract
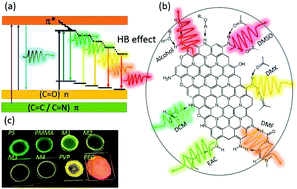
Carbon dots (CDs) have emerged as novel fluorescent probes due to their remarkable optical properties; however, red emission is still rare, has a relatively low efficiency, and its mechanism remains ambiguous. Herein, relatively efficient red-emission CDs based on p-phenylenediamine were prepared through various solvothermal means, where the highest quantum yield approached 41.1% in n-amyl alcohol, which was the most efficient quantum yield reported to date. Various structural characterizations were performed and confirmed that the red emission originated from the molecular states consisting of a nitrogen-containing organic fluorophore. The CDs were dispersed in different organic solvents and showed tunable emission, evolving from green to orange-red in aprotic solvents and a red emission in protic solvents. Further solvent correlation studies indicated that the hydrogen bond effect between the CDs and solvents was the main mechanism leading to the spectral shift. Accordingly, solid-state luminescent CDs–polymers were fabricated, which also demonstrated continuously tunable emission properties. This work opens a new window for recognizing the generation of tunable and red-emission CDs.


 Please wait while we load your content...
Please wait while we load your content...