Investigating the magnitude and source of orientation-dependent interactions between TiO2 crystal surfaces†
Abstract
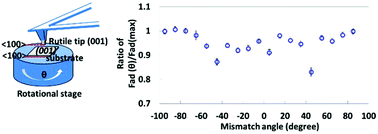
The oriented attachment (OA) of nanocrystals is a widely recognized non-classical crystallization mechanism. A fundamental understanding of the forces that governs the dynamics of particle movement, co-alignment, and attachment is needed to control crystal growth by OA. However, much remains unknown about the forces in the long range and molecular detail (such as the interfacial structure) effects in the short range, particularly in liquid suspensions. Using atomic force microscopy-based dynamic force spectroscopy to directly measure the adhesive force between two rutile TiO2 (001) crystal surfaces as a function of the lattice mismatch angle in water, we show that the forces exhibit 90° periodicity with respect to the lattice mismatch angle, which is generally consistent with the square-lattice arrangement of Ti4+ centers on the rutile TiO2 (001) surface. van der Waals and hydrogen bonding are the origin of adhesive forces. Molecular dynamics simulations that incorporate relevant molecular details provide a qualitative explanation for the observed orientation-dependence and suggest that hydrogen bonding is predicted to be the main source of the forces in a short range.


 Please wait while we load your content...
Please wait while we load your content...