A redox-controlled electrolyte for plasmonic enhanced dye-sensitized solar cells†
Abstract
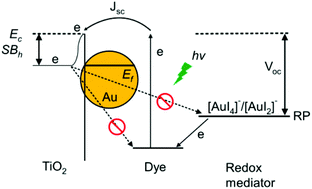
Plasmonic enhanced dye-sensitized solar cells (DSSCs) with metallic nanostructures suffer from corrosion problems, especially with the presence of the iodine/triiodide redox couple in the electrolyte. Herein, we introduce an alternative approach by compensating the corrosion with a modified liquid electrolyte. In contrast to the existing method of surface preservation for plasmonic nanostructures, the redox-controlled electrolyte (RCE) contains iodoaurate intermediates, i.e. gold(I) diiodide (AuI2−) and gold(III) tetraiodide (AuI4−) with optimal concentrations, such that these intermediates are readily reduced to gold nanoparticles during the operation of DSSCs. As corrosion and redeposition of gold occur simultaneously, it effectively provides corrosion compensation to the plasmonic gold nanostructures embedded in the photoanode. Cycling tests of the specific amount of gold contents in the RCE of DSSCs support the fact that the dissolution and deposition of gold are reversible and repeatable. This gold deposition on the TiO2 photoanode results in forming a Schottky barrier (SB) at the metal–semiconductor interface and effectively inhibits the recombination of electron–hole pairs. Therefore, the RCE increases the short-circuit current, amplifies the open-circuit voltage, and reduces the impedance of the TiO2/dye interface. The power conversion efficiency of DSSCs was improved by 57% after incorporating the RCE.


 Please wait while we load your content...
Please wait while we load your content...