A hierarchically porous nickel–copper phosphide nano-foam for efficient electrochemical splitting of water†
Abstract
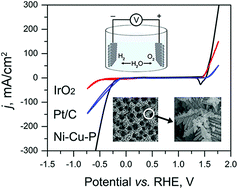
Electrochemical splitting of water to produce oxygen (O2) and hydrogen (H2) through a cathodic hydrogen evolution reaction (HER) and an anodic oxygen evolution reaction (OER) is a promising green approach for sustainable energy supply. Here we demonstrated a porous nickel-copper phosphide (NiCuP) nano-foam as a bifunctional electrocatalyst for highly efficient total water splitting. Prepared from a bubble-templated electrodeposition method and subsequent low-temperature phosphidization, NiCuP has a hierarchical pore structure with a large electrochemical active surface area. To reach a high current density of 50 mA cm−2, it requires merely 146 and 300 mV with small Tafel slopes of 47 and 49 mV dec−1 for HER and OER, respectively. The total water splitting test using NiCuP as both the anode and cathode showed nearly 100% Faradic efficiency and surpassed the performances of electrode pairs using commercial Pt/C and IrO2 catalysts under our test conditions. The high activity of NiCuP can be attributed to (1) the conductive NiCu substrates, (2) a large electrochemically active surface area together with a combination of pores of different sizes, and (3) the formation of active Ni/Cu oxides/hydroxides while keeping a portion of more conductive Ni/Cu phosphides in the nano-foam. We expect the current catalyst to enable the manufacturing of affordable water splitting systems.


 Please wait while we load your content...
Please wait while we load your content...