Titanocene dichloride (Cp2TiCl2) as a precursor for template-free fabrication of hollow TiO2 nanostructures with enhanced photocatalytic hydrogen production†
Abstract
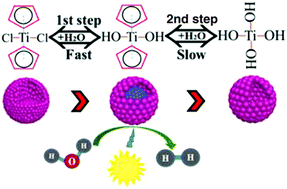
A one-pot and template-free strategy for synthesizing hollow TiO2 nanostructures (HTSs) is developed by using titanocene dichloride as a titanium source, acetone as a solvent, and ammonia as a basic source. Transmission electron microscopy (TEM) observations demonstrate that the morphology transformation undergoes solid, yolk–shell and then hollow structures, typical of an Ostwald ripening process. Comparative experiments suggest that the mismatched hydrolysis rate of chloride anion and organic cyclopentadiene in unique titanocene dichloride (Cp2TiCl2) molecules should be responsible for the formation of HTSs. The TiO2 nanostructures exhibit controllable morphologies and tunable sizes by mainly adjusting the amounts of the titanium precursor or ammonia. The HTSs show much improved photocatalytic performance as compared with samples of other morphologies in water splitting application, due to the remarkably increased surface area and active sites, and enhanced mass transfer. Our findings reported herein may offer new perspectives in materials chemistry, and energy- and environment-related applications.

 Please wait while we load your content...
Please wait while we load your content...