Understanding carbohydrate–protein interactions using homologous supramolecular chiral Ru(ii)-glyconanoclusters†
Abstract
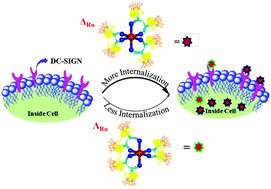
Multivalent glycodendrimers make promising tools to tackle the basic and translational research in the field of carbohydrate-mediated interactions. Despite advances in glycodendrimers and glycopolymers, the multivalent probes available to date are still far from being ideal biological mimics. This work demonstrates the inherent chirality of glycodendrimers to be one of the promising factors to generate different spatial carbohydrate micro-environments to modulate specific carbohydrate–protein interactions. By exploiting the host–guest strategy, chiral Ru(II) complexes (Δ and Λ) and mannose capped β-cyclodextrin (β-CD), we generated a library of homologous metallo-glycodendrimers (MGDs) with sizes of 50–70 nm. These nanoclusters can enantioselectively bind to specific C-type lectins and displayed selectivity in cellular uptake. We also discovered their potential clathrin-mediated endocytotic pathway in DC-SIGN and SIGNR3-transfected cell lines. Finally, in vivo biodistribution and sequestration of MGDs was determined to understand the role of chirality mediated spatial arrangement in carbohydrate-mediated interactions.

 Please wait while we load your content...
Please wait while we load your content...