Competition between self-inclusion and drug binding explains the pH dependence of the cyclodextrin drug carrier – molecular modelling and electrochemistry studies†
Abstract
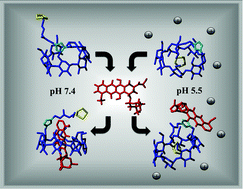
A non-toxic lipoic acid derivative of β cyclodextrin (βCDLip) with an electron-rich aromatic linker was studied as a carrier for the drug doxorubicin with the aim of decreasing the toxic side effects of this drug. The modified cyclodextrin strengthened the drug binding and differentiated the complex-forming ability with dependence on pH. The stability constants of the complexes were evaluated by voltammetry and spectrofluorometry. Molecular modelling provided deeper insight into the nature of the ligand structure itself and the drug–ligand interactions, showing the different contributions of the self-inclusion of the ligand substituent at different pH values. As a result, the modes of interaction of βCDLip with the drug and factors affecting the stabilities of the complex under the pH conditions of healthy and tumour cells could be discovered and explained.

 Please wait while we load your content...
Please wait while we load your content...