Enhanced protein internalization and efficient endosomal escape using polyampholyte-modified liposomes and freeze concentration†
Abstract
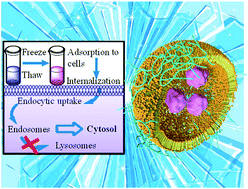
Here we show a new strategy for efficient freeze concentration-mediated cytoplasmic delivery of proteins, obtained via the endosomal escape property of polyampholyte-modified liposomes. The freeze concentration method successfully induces the efficient internalization of proteins simply by freezing cells with protein and nanocarrier complexes. However, the mechanism of protein internalization remains unclear. Here, we designed a novel protein delivery carrier by modifying liposomes through incorporating hydrophobic polyampholytes therein. These complexes were characterized for particle size, encapsulation efficiency, and cytotoxicity. Flow cytometry and microscopic analysis showed that the adsorption and internalization of protein-loaded polyampholyte-modified liposomes after freezing were enhanced compared with that observed in unfrozen complexes. Inhibition studies demonstrated that the internalization mechanism differs between unmodified and polyampholyte-modified liposomes. Furthermore, polyampholyte-modified liposomes exhibited high efficacy in facilitating endosomal escape to enhance protein delivery to the cytoplasm with low toxicity. These results strongly suggest that the freeze concentration-based strategy could be widely utilised for efficient cargo delivery into the cytoplasm in vitro not only in cancer treatment but also for gene therapy as well.

 Please wait while we load your content...
Please wait while we load your content...