Sequential protein unfolding through a carbon nanotube pore†
Abstract
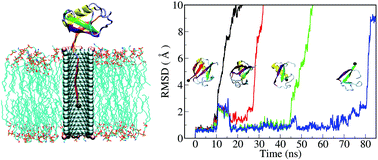
An assortment of biological processes, like protein degradation and the transport of proteins across membranes, depend on protein unfolding events mediated by nanopore interfaces. In this work, we exploit fully atomistic simulations of an artificial, CNT-based nanopore to investigate the nature of ubiquitin unfolding. With one end of the protein subjected to an external force, we observe non-canonical unfolding behaviour as ubiquitin is pulled through the pore opening. Secondary structural elements are sequentially detached from the protein and threaded into the nanotube, interestingly, the remaining part maintains native-like characteristics. The constraints of the nanopore interface thus facilitate the formation of stable “unfoldon” motifs above the nanotube aperture that can exist in the absence of specific native contacts with the other secondary structure. Destruction of these unfoldons gives rise to distinct force peaks in our simulations, providing us with a sensitive probe for studying the kinetics of serial unfolding events. Our detailed analysis of nanopore-mediated protein unfolding events not only provides insight into how related processes might proceed in the cell, but also serves to deepen our understanding of structural arrangements which form the basis for protein conformational stability.

 Please wait while we load your content...
Please wait while we load your content...