Self-assembly of like-charged nanoparticles into microscopic crystals†
Abstract
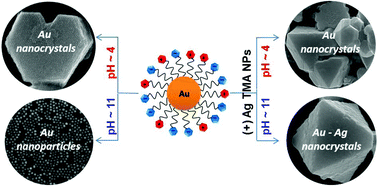
Like-charged nanoparticles, NPs, can assemble in water into large, faceted crystals, each made of several million particles. These NPs are functionalized with mixed monolayers comprising ligands terminating in carboxylic acid group ligands as well as positively charged quaternary ammonium ligands. The latter groups give rise to electrostatic interparticle repulsions which partly offset the hydrogen bonding between the carboxylic acids. It is the balance between these two interactions that ultimately enables self-assembly. Depending on the pH, the particles can crystallize, form aggregates, remain unaggregated or even – in mixtures of two particle types – can “choose” whether to crystallize with like-charged or oppositely charged particles.

 Please wait while we load your content...
Please wait while we load your content...