Charge transfer effects on the chemical reactivity of PdxCu1−x nanoalloys†
Abstract
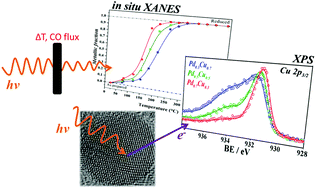
This work reports on the synthesis and characterization of PdxCu1−x (x = 0.7, 0.5 and 0.3) nanoalloys obtained via an eco-friendly chemical reduction method based on ascorbic acid and trisodium citrate. The average size of the quasi-spherical nanoparticles (NPs) obtained by this method was about 4 nm, as observed by TEM. The colloids containing different NPs were then supported on carbon in order to produce powder samples (PdxCu1−x/C) whose electronic and structural properties were probed by different techniques. XRD analysis indicated the formation of crystalline PdCu alloys with a nanoscaled crystallite size. Core-level XPS results provided a fingerprint of a charge transfer process between Pd and Cu and its dependency on the nanoalloy composition. Additionally, it was verified that alloying was able to change the NP's reactivity towards oxidation and reduction. Indeed, the higher the amount of Pd in the nanoalloy, less oxidized are both the Pd and the Cu atoms in the as-prepared samples. Also, in situ XANES experiments during thermal treatment under a reducing atmosphere showed that the temperature required for a complete reduction of the nanoalloys depends on their composition. These results envisage the control at the atomic level of novel catalytic properties of such nanoalloys.

 Please wait while we load your content...
Please wait while we load your content...