MoV2O8 nanostructures: controlled synthesis and lithium storage mechanism†
Abstract
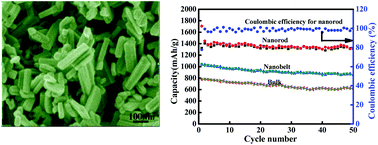
A facile two-step strategy involving a solvothermal method and a subsequent calcining treatment was successfully developed for the preparation of MoV2O8 nanorods in the absence of any surfactants. Acetic acid was chosen as the solvent to provide an acidic environment. The as-synthesized MoV2O8 nanorods were evaluated as an anode material in lithium ion batteries, which showed excellent lithium storage performance in terms of its specific capacity, rate performance, and cycling stability. It could deliver a specific capacity of over 1325 mA h g−1 after 50 cycles at 0.2 A g−1, which is much higher than that of bulk MoV2O8 (617 mA h g−1). When the cell was cycled at a current density as high as 10.0 A g−1, it still maintained a high specific capacity of around 570 mA h g−1. The phase transformation, intercalation–deintercalation and partial redox processes are responsible for the lithium storage mechanism of MoV2O8 based on ex situ X-ray diffraction, X-ray photo electron spectroscopy and transmission electron microscopy studies, highlighting a new lithium storage mechanism for ternary metal oxides.

 Please wait while we load your content...
Please wait while we load your content...