Organic crystal-binding peptides: morphology control and one-pot formation of protein-displaying organic crystals†
Abstract
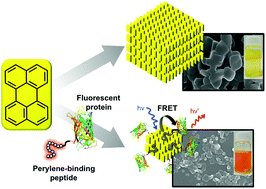
Crystalline assemblies of fluorescent molecules have different functional properties than the constituent monomers, as well as unique optical characteristics that depend on the structure, size, and morphological homogeneity of the crystal particles. In this study, we selected peptides with affinity for the surface of perylene crystal particles by exposing a peptide-displaying phage library in aqueous solution to perylene crystals, eluting the surface-bound phages by means of acidic desorption or liquid–liquid extraction, and amplifying the obtained phages in Escherichia coli. One of the perylene-binding peptides, PeryBPb1: VQHNTKYSVVIR, selected by this biopanning procedure induced perylene molecules to form homogenous planar crystal nanoparticles by means of a poor solvent method, and fusion of the peptide to a fluorescent protein enabled one-pot formation of protein-immobilized crystalline nanoparticles. The nanoparticles were well-dispersed in aqueous solution, and Förster resonance energy transfer from the perylene crystals to the fluorescent protein was observed. Our results show that the crystal-binding peptide could be used for simultaneous control of perylene crystal morphology and dispersion and protein immobilization on the crystals.

 Please wait while we load your content...
Please wait while we load your content...