Ni12P5 nanoparticles decorated on carbon nanotubes with enhanced electrocatalytic and lithium storage properties†
Abstract
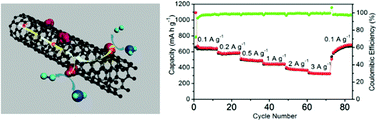
Transition-metal phosphides (TMPs) have been proved to be of great importance in electrochemical energy conversion and Li-ion storage. In this work, we have designed a useful one-pot hot-solution colloidal synthetic route for synthesizing a new kind of unique hybrid nanostructures (the Ni12P5/CNT nanohybrids) by direct in situ growth of Ni12P5 nanocrystals onto oxidized multiwall carbon nanotubes (CNTs). The CNTs can improve the conductivity of the hybrids and effectively prevent the aggregation of Ni12P5 nanoparticles in the cycle process. When they are evaluated as a novel non-noble-metal hydrogen evolution reaction (HER) catalyst operating in acidic electrolytes, the Ni12P5/CNT nanohybrids exhibit an onset overpotential as low as 52 mV and a Tafel slope of 56 mV dec−1 and only require overpotentials of 65 and 129 mV to attain current densities of 2 and 10 mA cm−2, respectively. Moreover, they also exhibit enhanced electrochemical performance for lithium-ion batteries serving as an anode material; the Ni12P5/CNT nanohybrids show a high capacity, excellent cycling stability and good rate performance. Their unusual properties arise from a synergetic effect between Ni12P5 and CNTs.

 Please wait while we load your content...
Please wait while we load your content...