MoO2–CoO coupled with a macroporous carbon hybrid electrocatalyst for highly efficient oxygen evolution†
Abstract
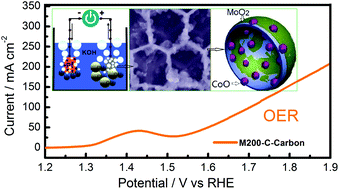
Cost-effective electrocatalysts for oxygen evolution reactions are attractive for energy conversion and storage processes. A high-performance oxygen evolution reaction (OER) electrocatalyst composed of 3D ordered microporous carbon and a MoO2 skeleton modified by cobalt oxide nanoparticles (MoO2–CoO–Carbon) is produced through a template method. This unique 3DOM structure finely combines the larger surface area of the 3D carbon skeleton and MoO2 as well as stablizes anchoring sites for CoO nanocrystals on the skeleton. The synergistic effect between the catalytic activity between MoO2 and CoO as well as the enhanced electron transport arising from the carbon skeleton contributed to superior electrocatalytic OER properties of MoO2–CoO–Carbon. The M200–C–Carbon hybrid with an overpotential as low as 0.24 V is among the best reported Mo-based OER catalysts. Moreover, the turnover frequency at an overpotential of 0.35 V is 6 times as high as that of commercial RuO2.

 Please wait while we load your content...
Please wait while we load your content...