Influence of electroosmotic flow on the ionic current rectification in a pH-regulated, conical nanopore†
Abstract
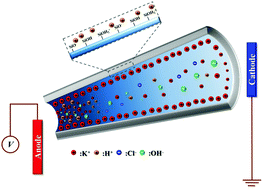
The ionic current rectification (ICR) is studied theoretically by considering a pH-regulated, conical nanopore. In particular, the effect of electroosmotic flow (EOF), which was often neglected in previous studies, is investigated by solving a set of coupled Poisson, Nernst–Planck, and Navier–Stokes equations. The behaviors of ICR under various conditions are examined by varying solution pH, bulk ionic concentration, and applied electric potential bias. We show that the EOF effect is significant when the bulk ionic concentration is medium high, the pH is far away from the iso-electric point, and the electric potential bias is high. The percentage deviation in the current rectification ratio arising from neglecting the EOF effect can be on the order of 100%. In addition, the behavior of the current rectification ratio at a high pH taking account of EOF is different both qualitatively and quantitatively from that without taking account of EOF.

 Please wait while we load your content...
Please wait while we load your content...