Water–methanol separation with carbon nanotubes and electric fields†
Abstract
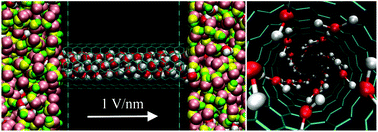
Methanol is used in various applications, such as fuel for transportation vehicles, fuel cells, and in chemical industrial processes. Conventionally, separation of methanol from aqueous solution is by distillation. However, this method consumes a large amount of energy; hence development of a new method is needed. In this work, molecular dynamics simulations are performed to investigate the effect of an electric field on water–methanol separation by carbon nanotubes (CNTs) with diameters of 0.81 to 4.07 nm. Without an electric field, methanol molecules fill the CNTs in preference to water molecules. The preference of methanol to occupy the CNTs over water results in a separation effect. This separation effect is strong for small CNT diameters and significantly decreases with increasing diameter. In contrast, under an electric field, water molecules strongly prefer to occupy the CNTs over methanol molecules, resulting in a separation effect for water. More interestingly, the separation effect for water does not decrease with increasing CNT diameter. Formation of water structures in CNTs induced by an electric field has an important role in the separation of water from methanol.

 Please wait while we load your content...
Please wait while we load your content...