Germanium nanocrystals as luminescent probes for rapid, sensitive and label-free detection of Fe3+ ions†
Abstract
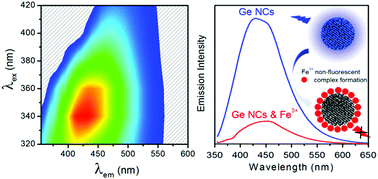
Luminescent water-soluble germanium nanocrystals (Ge NCs) have been developed as a fluorescent sensing platform for the highly selective and sensitive detection of Fe3+via quenching of their strong blue luminescence, without the need for analyte-specific labelling groups. The amine-terminated Ge NCs were separated into two discrete size fractions with average diameters of 3.9 ± 0.4 nm and 6.8 ± 1.8 nm using centrifugation. The smaller 3.9 nm NCs possessed a strong blue luminescence, with an average lifetime of 6.1 ns and a quantum yield (QY) of 21.5%, which is strongly influenced by solution pH. In contrast, 6.8 nm NCs exhibited a green luminescence with a longer lifetime of 7.8 ns and lower QY (6.2%) that is insensitive to pH. Sensitive detection of Fe3+ was successfully demonstrated, with a linear relationship between luminescence quenching and Fe3+ concentration observed from 0–800 μM, with a limit of detection of 0.83 μM. The Ge NCs show excellent selectivity toward Fe3+ ions, with no quenching of the fluorescence signal induced by the presence of Fe2+ ions, allowing for solution phase discrimination between ions of the same element with different formal charges. The luminescence quenching mechanism was confirmed by static and time-resolved photoluminescence spectroscopies, while the applicability for this assay for detection of Fe3+ in real water samples was successfully demonstrated.

 Please wait while we load your content...
Please wait while we load your content...