Morphogenesis mechanisms in the solvothermal synthesis of BaTiO3 from titanate nanorods and nanotubes†
Abstract
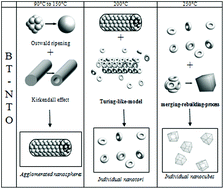
A rich variety of single crystalline BaTiO3 (BT) nanostructures have been synthesized by two different routes using titanate nanorods and nanotubes as precursors. Free standing, mixed or agglomerated nanotori, solid or hollow nanospheres and nanocubes were obtained. A careful analysis of the shape evolution of the resulting BT nano-objects obtained with both types of precursors and different parameters (precursor composition and shape, temperature, Ba/Ti molar ratio) allowed an improved understanding of the nanostructure formation. The morphogenesis models at play such as Ostwald ripening and the Kirkendall effect have been identified. Other mechanisms hereafter called the self and merging rebuilding processes and a tentative Turing-reaction–diffusion-model are proposed to explain the formation of these obtained nanoparticles.

 Please wait while we load your content...
Please wait while we load your content...