Catalyst engineering for lithium ion batteries: the catalytic role of Ge in enhancing the electrochemical performance of SnO2(GeO2)0.13/G anodes†
Abstract
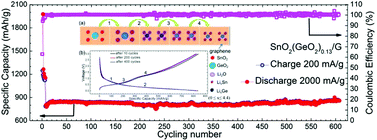
The catalytic role of germanium (Ge) was investigated to improve the electrochemical performance of tin dioxide grown on graphene (SnO2/G) nanocomposites as an anode material of lithium ion batteries (LIBs). Germanium dioxide (GeO2) and SnO2 nanoparticles (<10 nm) were uniformly anchored on the graphene sheets via a simple single-step hydrothermal method. The synthesized SnO2(GeO2)0.13/G nanocomposites can deliver a capacity of 1200 mA h g−1 at a current density of 100 mA g−1, which is much higher than the traditional theoretical specific capacity of such nanocomposites (∼702 mA h g−1). More importantly, the SnO2(GeO2)0.13/G nanocomposites exhibited an improved rate, large current capability (885 mA h g−1 at a discharge current of 2000 mA g−1) and excellent long cycling stability (almost 100% retention after 600 cycles). The enhanced electrochemical performance was attributed to the catalytic effect of Ge, which enabled the reversible reaction of metals (Sn and Ge) to metals oxide (SnO2 and GeO2) during the charge/discharge processes. Our demonstrated approach towards nanocomposite catalyst engineering opens new avenues for next-generation high-performance rechargeable Li-ion batteries anode materials.

 Please wait while we load your content...
Please wait while we load your content...