Redox properties of a single (7,5)single-walled carbon nanotube determined by an in situ photoluminescence spectroelectrochemical method
Abstract
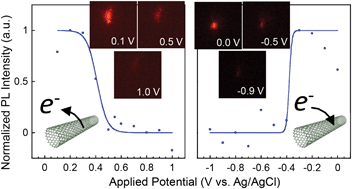
The determination of electronic states of single-walled carbon nanotubes (SWNTs) has been a central issue in science and nanotechnology of carbon nanotubes. We here describe the oxidation and reduction potentials of a single SWNT determined by in situ photoluminescence (PL) spectroelectrochemical measurements. By PL imaging and single SWNT PL spectroscopy, the stepwise quenching behavior of the PL from a single (7,5)SWNT was detected as the outer-applied potentials increased. Based on the analysis of the obtained potential-dependent PL plots using the Nernst equation, the oxidation and reduction potentials of the (7,5) tube are successfully determined as 0.41 V and −0.38 V vs. Ag/AgCl, respectively, which shift from those of the bulk (7,5)SWNTs. We further observed a PL blueshift and narrowing of the line width as the external-applied potential to the single SWNT increases. The present results are important for understanding the electronic properties of a single (n,m)SWNT and its applications.

 Please wait while we load your content...
Please wait while we load your content...