Synthesis of Bi nanowire networks and their superior photocatalytic activity for Cr(vi) reduction†
Abstract
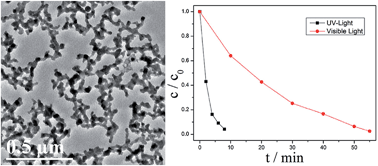
Interconnected Bi nanowire networks were synthesized for the first time via a solvothermal route by using ethylene glycol (EG) as both a solvent and a reducing agent, and citric acid (CA) as a stabilizing agent at a molar ratio of CA/Bi3+ = 5. Among various reaction conditions including the temperature, reaction time and precursor concentration, the molar ratio of CA/Bi3+ was the dominant experimental parameter to influence the morphology and structures of the Bi crystals. Highly dispersed Bi microspheres and network-like Bi thick wires were obtained if the molar ratio of CA/Bi3+ was changed to 2.5 and 10, respectively. As compared to other additives including trisodium citrate, cetyltrimethylammonium bromide (CTAB) and oxalic acid, good solubility of CA in EG together with its coordination effect played a crucial role in the formation of network-like Bi nanowires. The Bi nanowire networks exhibited excellent photocatalytic performance for Cr(VI) reduction. Cr(VI) was completely reduced to less toxic Cr(III) after 8 min and 55 min of UV and visible-light irradiation, respectively.

 Please wait while we load your content...
Please wait while we load your content...