Towards graphene bromide: bromination of graphite oxide†
Abstract
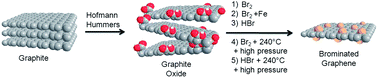
Halogenated graphene derivatives are interesting for their outstanding physical and chemical properties. In this paper, we present various methods for the synthesis of brominated graphene derivatives by the bromination of graphite oxides. Graphite oxides, prepared according to either the Hummers or Hofmann method, were brominated using bromine or hydrobromic acid under reflux or in an autoclave at elevated temperatures and pressures. The influence of both graphite oxide precursors on the resulting brominated graphenes was investigated by characterization of the graphenes, which was carried out using various techniques, including SEM, SEM-EDS, high-resolution XPS, FTIR, STA and Raman spectroscopy. In addition, the resistivity of the brominated graphenes was measured and the electrochemical properties were investigated by cyclic voltammetry. Although the brominated graphenes were structurally similar, they had remarkably different bromine concentrations. The most highly brominated graphene (bromine concentration above 26 wt%) exhibited a C/O ratio above 44 and partial hydrogenation. Brominated graphenes with such properties could be used for reversible bromine storage or as a starting material for further chemical modifications.

 Please wait while we load your content...
Please wait while we load your content...