Highly branched platinum nanolance assemblies by polyallylamine functionalization as superior active, stable, and alcohol-tolerant oxygen reduction electrocatalysts†
Abstract
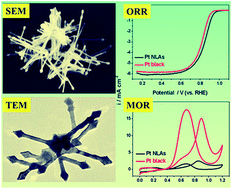
The chemical functionalization of platinum (Pt) nanostructures is becoming a new trend in electrocatalysts designs. Meanwhile, highly branched Pt nanostructures are highly exciting electrocatalysts with high activity and stability owing to their specific physical and chemical properties. In this work, the polyallylamine (PAH) functionalized Pt nanolance assemblies (Pt NLAs) have been successfully synthesized by chemical reduction of PAH–PtII complex using formaldehyde (HCHO) in a two-phase water-complex system. The as-prepared Pt NLAs are highly branched and three-dimensionally (3D) interconnected nanostructures, which are composed of many long Pt nanolances in various directions. PAH functionalization improves the electrocatalytic activity of the Pt NLAs for an oxygen reduction reaction (ORR) because of high interface proton concentration on the Pt surface and excellent anti-oxidation ability of the Pt nanolances. Meanwhile, the PAH molecules bound on the Pt NLAs surface act as barrier networks to restrain accessibility of alcohol, exhibiting a high ORR selectivity. In addition, the PAH functionalized Pt NLAs show excellent durability for the ORR due to their particular 3D interconnected structure. The work demonstrates that the PAH functionalized Pt NLAs are indeed promising cathodic electrocatalysts for practical application in direct alcohol fuel cells.

 Please wait while we load your content...
Please wait while we load your content...