Theoretical aspects of WS2 nanotube chemical unzipping
Abstract
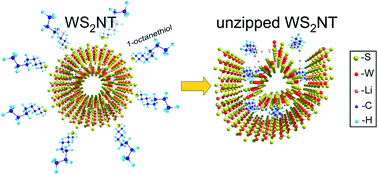
Theoretical analysis of experimental data on unzipping multilayered WS2 nanotubes by consequent intercalation of lithium atoms and 1-octanethiol molecules [C. Nethravathi, et al., ACS Nano, 2013, 7, 7311] is presented. The radial expansion of the tube was described using continuum thin-walled cylinder approximation with parameters evaluated from ab initio calculations. Assuming that the attractive driving force of the 1-octanethiol molecule is its reaction with the intercalated Li ions ab initio calculations of a 1-octanethiol molecule bonding with Li+ were carried out. In addition, the non-chemical interactions of the 1-octanethiol dipole with an array of positive point charges representing Li+ were taken into account. Comparing between the energy gain from these interactions and the elastic strain energy of the nanotube allows us to evaluate a value for the tube wall deformation after the implantation of 1-octanethiol molecules. The ab initio molecular dynamics simulation confirmed our estimates and demonstrated that a strained WS2 nanotube, with a decent concentration of 1-octanethiol molecules, should indeed be unzipped into the WS2 nanoribbon.

 Please wait while we load your content...
Please wait while we load your content...