Metallic influence on the atomic structure and optical activity of ligand-protected nanoparticles: a comparison between Ag and Au
Abstract
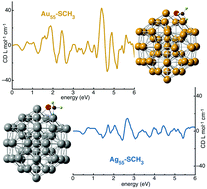
Using time-perturbed density functional theory the optical activity of metal–thiolate compounds formed by highly symmetric Ag and Au nanoparticles (NPs) and a methyl-thiol molecule is studied after performing atomic optimizations and electronic calculations upon adsorption. Many different sites and orientations of the adsorbed molecule on icosahedral Ag and Au NPs of 55 atoms are considered. Upon molecular adsorption atomic distortions on Au NPs are induced while not on Ag, which causes higher molecular adsorption energies in Au than in Ag. Structural distortions and the specific molecular adsorption site and orientation result in chiral metal–thiolate NPs. Ag and Au compounds with similar chirality, according to Hausdorff chirality measurements, show different optical activity signatures, where circular dichroism spectra of Au NPs are more intense. These dissimilarities are attributed in part to the differences in the electronic density of states, which are a consequence of relativistic effects and the atomic distortion. It is concluded that the optical activity of Ag and Au compounds is due to different mechanisms, while in Au it is mainly due to the atomic distortion of the metallic NPs induced after molecular adsorption, in Ag it is defined by the adsorption site and molecular orientation with respect to the NP symmetry.

 Please wait while we load your content...
Please wait while we load your content...