Mo polyoxometalate nanoclusters capable of inhibiting the aggregation of Aβ-peptide associated with Alzheimer's disease†
Abstract
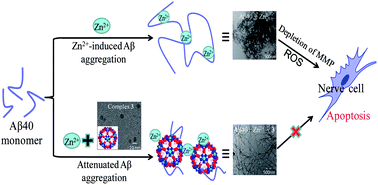
A neuropathological hallmark of Alzheimer's disease (AD) is aggregation of a forty-residue peptide known as amyloid beta forty (Aβ40). While past work has indicated that blocking Aβ40 aggregation could be an effective strategy for the treatment of AD, developing therapies with this goal has been met with limited success. Polyoxometalates (POMs) have been previously investigated for their anti-viral and anti-tumoral properties and we report here that three representative POM nanoclusters have been synthesized for use against Aβ40 aggregation. Through the use of thioflavin T fluorescence, turbidity, circular dichroism spectroscopy, and transmission electron microscopy (TEM), we found that all three POM complexes can significantly inhibit both natural Aβ40 self-aggregation and metal-ion induced Aβ40 aggregation. We also evaluated the protective effect of POM complexes on Aβ40-induced neurotoxicity in cultured PC12 cells and found that treatment with POM complexes can elevate cell viability, decrease levels of intracellular reactive oxygen species, and stabilize mitochondrial membrane potential. These findings indicate that all three representative POM complexes are capable of inhibiting Aβ40 aggregation and subsequent neurotoxicity. While a complete mechanistic understanding remains to be elucidated, the synthesized POM complexes may work through a synergistic interaction with metal ions and Aβ40. These data indicate that POM complexes have high therapeutic potential for use against one of the primary neuropathological features of AD.

 Please wait while we load your content...
Please wait while we load your content...