Endohedral metal or a fullerene cage based oxidation? Redox duality of nitride clusterfullerenes CexM3−xN@C78–88 (x = 1, 2; M = Sc and Y) dictated by the encaged metals and the carbon cage size†
Abstract
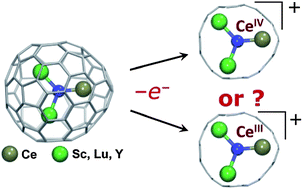
Redox behavior of endohedral metallofullerenes, in particular their oxidation process, can be classified as a fullerene-based or endohedral species-based process according to the mechanism of the electron transfer. Here we report on the phenomenon of the strain-driven electrochemical behavior achieved by encapsulating the cerium-containing clusters into a series of carbon cages ranging from C78 to C88. The Ce-based mixed metal nitride clusterfullerenes CexM3−xN@C2n (x = 1, 2; M = Sc or Y; 2n = 78–88) were synthesized and characterized. The magnitude of the inherent strain caused by the limited inner space of the carbon cage for the relatively large nitride clusters can be varied by choosing different scaffold metals (Sc, Lu, or Y) to tailor the size of the encaged CexM3−xN cluster and by matching the nitride cluster with different fullerene cages in the size range from C78 to C88. The redox properties of CexM3−xN@C2n were investigated by cyclic and square wave voltammetry. The mechanism of the electrochemical oxidation of Ce-based mixed metal nitride clusterfullerenes, in particular whether the fullerene-based oxidation or the CeIII → CeIV process is observed, is found to be dependent on the scaffold metal and the size of the fullerene cage. The endohedral oxidation of CeIII to CeIV was observed for a number of compounds as revealed by the negative shift of their oxidation potentials with respect to the values measured for the non-Ce analogues. Experimental studies are supported by DFT calculations. We conclude that the prerequisites for the Ce-based endohedral oxidation process are suitable inherent cluster-cage strain and sufficiently high oxidation potential of the fullerene cage.

 Please wait while we load your content...
Please wait while we load your content...