Microemulsion-based synthesis and electrochemical evaluation of different nanostructures of LiCoO2 prepared through sacrificial nanowire templates
Abstract
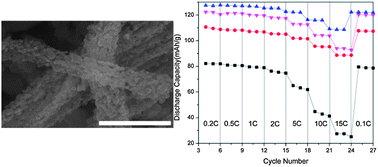
For the first time, we demonstrate the use of a microemulsion reaction to synthesize different nanostructures of LiCoO2 cathode material. By varying the annealing temperature and time, porous nanowires and nanoparticles of LiCoO2 are obtained. The electrochemical performances of these different nanostructures obtained under the respective annealing conditions are evaluated. It is shown that nanoparticles formed under the annealing condition of 700 °C, 1.5 h perform the best, delivering an initial capacity of around 135 mA h g−1, which is close to the theoretical capacity of LiCoO2, 140 mA h g−1. They also exhibit a capacity retention of around 93% by 100 cycles at 0.1 C. Comparisons are made between our LiCoO2 material obtained under different annealing conditions and those in the literature.

 Please wait while we load your content...
Please wait while we load your content...