ZnO nanorods/ZnS·(1,6-hexanediamine)0.5 hybrid nanoplates hierarchical heteroarchitecture with improved electrochemical catalytic properties for hydrazine†
Abstract
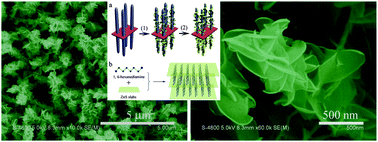
Novel hierarchical heteronanostructures of ZnO nanorods/ZnS·(HDA)0.5 (HDA = 1,6-hexanediamine) hybrid nanoplates on a zinc substrate are successfully synthesized on a large scale by combining hydrothermal growth (for ZnO nanorods) and liquid chemical conversion (for ZnS·(HDA)0.5 nanoplates) techniques. The formation of ZnS·(HDA)0.5 hybrid nanoplates branches takes advantage of the preferential binding of 1,6-hexanediamine on specific facets of ZnS, which makes the thickening rate much lower than the lateral growth rate. The ZnS·(HDA)0.5 hybrid nanoplates have a layered structure with 1,6-hexanediamine inserted into interlayers of wurtzite ZnS through the bonding of nitrogen. The number density and thickness of the secondary ZnS·(HDA)0.5 nanoplates can be conveniently engineered by variation of the sulfur source and straightforward adjustment of reactant concentrations such as 1,6-hexanediamine and the sulfur source. The fabricated ZnO/ZnS·(HDA)0.5 heteronanostructures show improved electrochemical catalytic properties for hydrazine compared with the primary ZnO nanorods. Due to its simplicity and efficiency, this approach could be similarly used to fabricate varieties of hybrid heterostructures made of materials with an intrinsic large lattice mismatch.

 Please wait while we load your content...
Please wait while we load your content...