Anomalous stability of graphene containing defects covered by a water layer†
Abstract
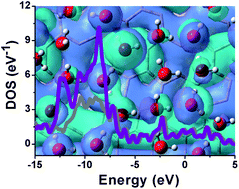
Defects are inevitably present in graphene and can alter its properties and thus its applications. Interestingly, we find that commonly observed Stone–Wales and double vacancy defects do not affect graphene's hydrophilic and hydrophobic properties and that an adsorbed single water layer does not noticeably affect the defect-containing graphene's electronic properties. Our findings are based on calculations using a density functional tight-binding theory. Specifically, we observe negligible alteration in the interaction strength (less than 0.1 kcal mol−1) between a single water layer and graphene upon the incorporation of the various types of defects, which indicates that graphene has relatively stable hydrophilic and hydrophobic properties. The presence of a single water layer causes only negligible changes in the energy gap and a small charge transfer to the aqueous layer (less than 0.1 e). The results indicate that the electronic properties of graphene are determined mainly by its own structural characteristics and are not considerably affected by the adsorbed water layer. Further electronic structure analysis reveals that the two commonly observed defects do not change the sp2 hybridization characteristics of the C atoms of graphene even in the water environment. Our results are significant for graphene studies and applications in areas such as life sciences and materials science where hydrophilic and hydrophobic properties and electronic properties are important.

 Please wait while we load your content...
Please wait while we load your content...