Hydrothermal synthesis of mesoporous silica spheres: effect of the cooling process†
Abstract
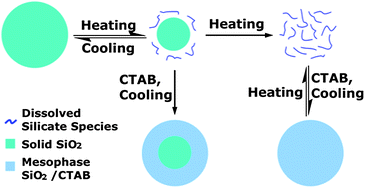
Hydrothermal methods have been widely used in the fabrication of silica-based micro-/nanomaterials. In this paper, we comprehensively investigated dissolution/regrowth kinetics of solid silica in alkaline media under relatively high temperature hydrothermal conditions (typically 180 °C). A decoupled dissolution and regrowth mechanism was proposed to explain the transformation of solid silica to mesoporous silica spheres in the presence of CTAB surfactant. Especially, we discovered that the “post-synthesis” sample cooling process plays a great role in the present hydrothermal process. The proposed mechanism can be utilized for the preparation of mesoporous silica spheres from various silica sources. Moreover, the mechanism is also applicable to other nonsurfactant hydrothermal processes.

 Please wait while we load your content...
Please wait while we load your content...