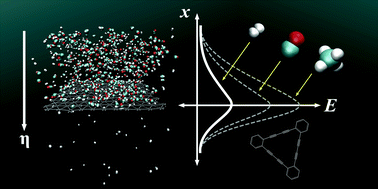
Graphdiyne, a recently synthesized one-atom-thick carbon allotrope, is atomistically porous – characterized by a regular “nanomesh” – and suggests application as a separation membrane for hydrogen purification. Here we report a full atomistic reactive molecular dynamics investigation to determine the selective diffusion properties of hydrogen (H2) amongst carbon monoxide (CO) and methane (CH4), a mixture otherwise known as syngas, a product of the gasification of renewable biomass (such as animal wastes). Under constant temperature simulations, we find the mass flux of hydrogen molecules through a graphdiyne membrane to be on the order of 7 to 10 g cm−2 s−1 (between 300 K and 500 K), with carbon monoxide and methane remaining isolated. Using a simple Arrhenius relation, we determine the energy required for permeation on the order of 0.11 ± 0.03 eV for single H2 molecules. We find that addition of marginal applied force (approximately 1 to 2 pN per molecule, representing a controlled pressure gradient, ΔP, on the order of 100 to 500 kPa) can successfully enhance the separation of hydrogen gas. Addition of larger driving forces (50 to 100 pN per molecule) is required to selectively filter carbon monoxide or methane, suggesting that, under near-atmospheric conditions, only hydrogen gas will pass such a membrane. Graphdiyne provides a unique, chemically inert and mechanically stable platform facilitating selective gas separation at nominal pressures using a homogeneous material system, without a need for chemical functionalization or the explicit introduction of molecular pores.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...