The synthesis of cardenolide and bufadienolide aglycones, and related steroids bearing a heterocyclic subunit†
Abstract
Covering: early studies through to March 2016
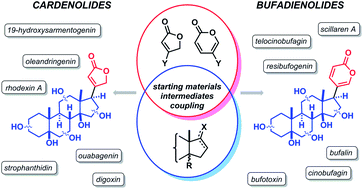
Cardenolides and bufadienolides constitute an attractive class of biologically active steroid derivatives which have been used for the treatment of heart disease in traditional remedies as well as in modern medicinal therapy. Due to their application as therapeutic agents and their unique molecular structures, bearing unsaturated 5- or 6-membered lactones (or other heterocycles) attached to the steroid core, cardio-active steroids have received great attention, which has intensified during the last decade, in the synthetic organic community. Advances in the field of cross-coupling reactions have provided a powerful tool for the attachment of lactone subunits to the steroid core. This current review covers a methodological analysis of synthetic efforts to cardenolide and bufadienolide aglycones. Special emphasis is given to cross-coupling reactions applied for the attachment of lactone subunits at sterically very hindered positions of the steroid core. The carefully selected partial and total syntheses of representative cardio-active steroids will also be presented to exemplify recent achievements (improvements) in the field.


 Please wait while we load your content...
Please wait while we load your content...