Emergence of diversity and stereochemical outcomes in the biosynthetic pathways of cyclobutane-centered marine alkaloid dimers
Abstract
Covering: up to 2016
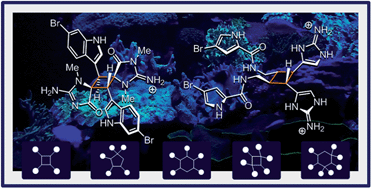
Dictazoles and sceptrins are singular metabolites of marine origin. The present dichotomic case study provides a comprehensive perspective on these cyclobutane-centered alkaloids and their respective families. Indeed, their upstream and downstream chemistry are both treated herein. Relevant isolation reports and bio-inspired total syntheses are used to decipher the currently admitted biosynthetic hypotheses as well as the emergence of diversity in the two series. This review proposes a transversal vision of the topic, where most aspects of natural product chemistry have a critical importance.

 Please wait while we load your content...
Please wait while we load your content...