Target identification of natural products and bioactive compounds using affinity-based probes
Abstract
Covering: 2010 to 2014.
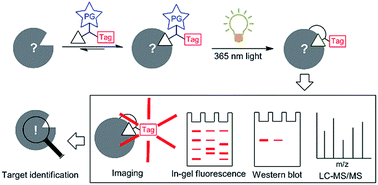
Advances in isolation, synthesis and screening strategies have made many bioactive substances available. However, in most cases their putative biological targets remain unknown. Herein, we highlight recent advances in target identification of natural products and bioactive compounds by using affinity-based probes. Aided by photoaffinity labelling, this strategy can capture potential cellular targets (on and off) of a natural product or bioactive compound in live cells directly, even when the compound–target interaction is reversible with moderate affinity. The knowledge of these targets may help uncover molecular pathways and new therapeutics for currently untreatable diseases. In this highlight, we will introduce the development of various photoactivatable groups, their synthesis and applications in target identification of natural products and bioactive compounds, with a focus on work done in recent years and from our laboratory. We will further discuss the strengths and weaknesses of each group and the outlooks for this novel proteome-wide profiling strategy.
- This article is part of the themed collection: Strategies for Cellular Target Identification of Natural Products

 Please wait while we load your content...
Please wait while we load your content...