Zampanolide and dactylolide: cytotoxic tubulin-assembly agents and promising anticancer leads
Abstract
Covering: through January 2014
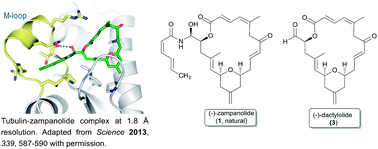
Zampanolide is a marine natural macrolide and a recent addition to the family of microtubule-stabilizing cytotoxic agents. Zampanolide exhibits unique effects on tubulin assembly and is more potent than paclitaxel against several multi-drug resistant cancer cell lines. A high-resolution crystal structure of αβ-tubulin in complex with zampanolide explains how taxane-site microtubule-stabilizing agents promote microtubule assemble and stability. This review provides an overview of current developments of zampanolide and its related but less potent analogue dactylolide, covering their natural sources and isolation, structure and conformation, cytotoxic potential, structure–activity studies, mechanism of action, and syntheses.

 Please wait while we load your content...
Please wait while we load your content...