Polyketide construction via hydrohydroxyalkylation and related alcohol C–H functionalizations: reinventing the chemistry of carbonyl addition
Abstract
Covering: up to the end of 2013
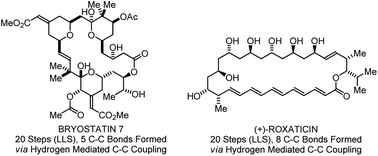
Despite the longstanding importance of polyketide natural products in human medicine, nearly all commercial polyketide-based drugs are prepared through fermentation or semi-synthesis. The paucity of manufacturing routes involving de novo chemical synthesis reflects the inability of current methods to concisely address the preparation of these complex structures. Direct alcohol C–H bond functionalization via “C–C bond forming transfer hydrogenation” provides a powerful, new means of constructing type I polyketides that bypasses stoichiometric use of chiral auxiliaries, premetallated C-nucleophiles, and discrete alcohol-to-aldehyde redox reactions. Using this emergent technology, total syntheses of 6-deoxyerythronolide B, bryostatin 7, trienomycins A and F, cyanolide A, roxaticin, and formal syntheses of rifamycin S and scytophycin C, were accomplished. These syntheses represent the most concise routes reported to any member of the respective natural product families.
- This article is part of the themed collection: Synthesis III

 Please wait while we load your content...
Please wait while we load your content...